

What Is A Serial Dilution Serial Vs Parallel Processing Once you understand these principles, you will be better able to design the dilutions you need for each specific case. It explains some principles for designing dilutions that give optimal results. Dilution is the process of making a less concentrated solution and dilution factor is the amount of solvent required to make a certain concentration of solution.
#SERIAL DILUTION VS PARALLEL DILUTION SERIES#
Preparing a series of calibration standards by this method reduces the amount of required time. Advantages of Serial Dilutions This section is not a recipe for your experiment. What is the advantage of performing a serial dilution instead of a single dilution?Įasier and Faster Preparation of Calibration Standards The errors introduced with each successive dilution drops proportionately with the solution concentration. Essential to direct dilution is the ability to accurately transfer extremely small volumes of stock solution, which is generally not possible with pipets. This figure shows only the first four concentrations via direct dilution. Remember that the smallest volume you can measure with the micropipettors is 2 L. The procedures of cell preparation and the. Serial Vs Parallel Dilution SECOND: Also use a serial dilution when the dilution factor is so large that the amount of stock solution needed to make the dilution in one step (using the formula C 1V 1 C 2V 2) is too small to measure accurately. The direct dilution method uses far less sample than the serial dilution method. Fluorimetric assay of serial dilution of adherent cells via DCFH-DA.Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale.įurthermore, is direct or serial dilution more accurate? A ten-fold serial dilution could be 1 M, 0.1 M, 0.01 M, 0.001 M. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. In pharmaceutical laboratories, serial dilution is performed to receive the necessary concentration of chemicals and compounds as this method is more effective than individual dilutions. A serial dilution is the stepwise dilution of a substance in solution.
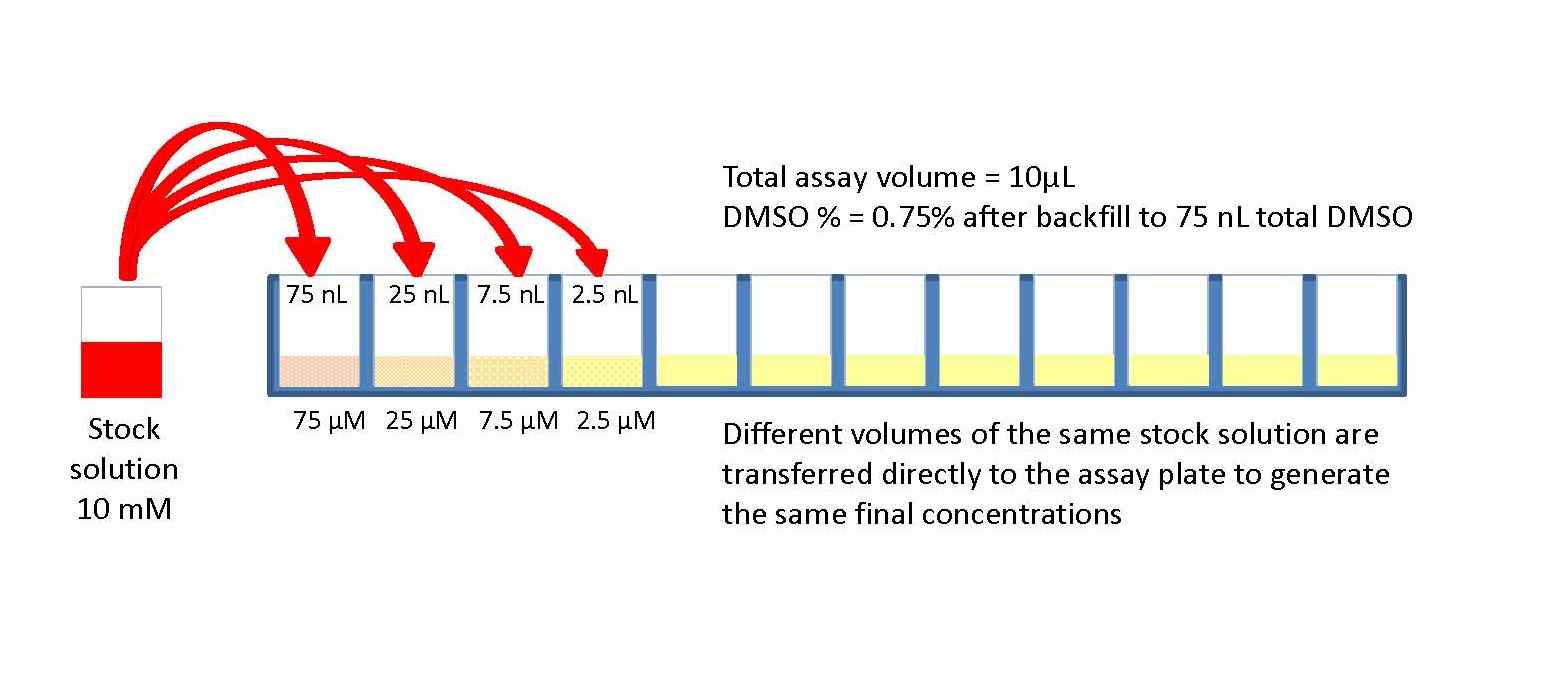
Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves).Īlso Know, what is serial dilution and why is it used? Serial dilution is the stepwise dilution of a substance in solution. In biochemistry, serial dilution is used to obtain the desired concentration of reagents and chemicals from a higher concentration. Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. Keeping this in consideration, what are the advantages of serial dilution? Clearly, accurate pipetting during preparation of serial dilutions is critical, because any deviation will propagate to all of the subsequent steps. It is much more accurate to make several smaller stepwise dilutions to reach a final concentration when the required reduction in concentration is large.


 0 kommentar(er)
0 kommentar(er)
